Topics
late
AI
Amazon
Image Credits:Andrew Brookes / Getty Images
Apps
Biotech & Health
Climate

Image Credits:Andrew Brookes / Getty Images
Cloud Computing
commercialism
Crypto
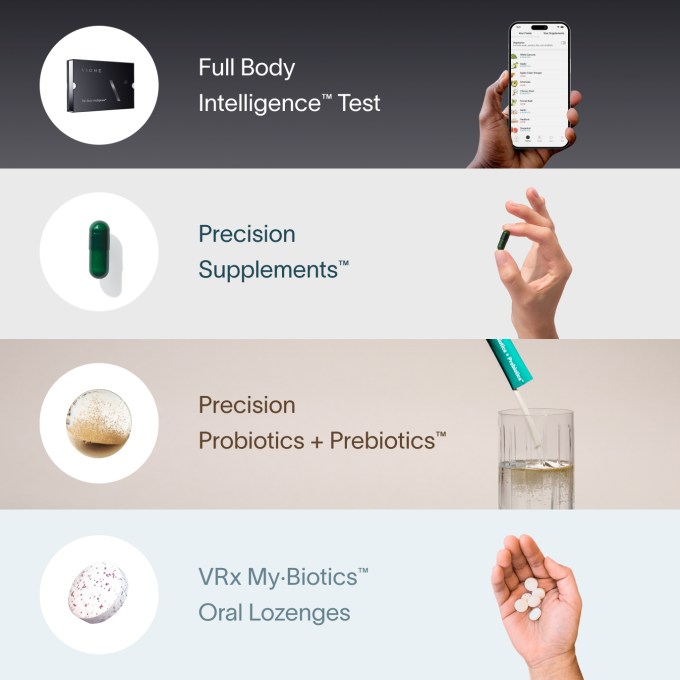
Viome’s front-page testing-through-supplements pitch.Image Credits:Viome
Enterprise
EVs
Fintech
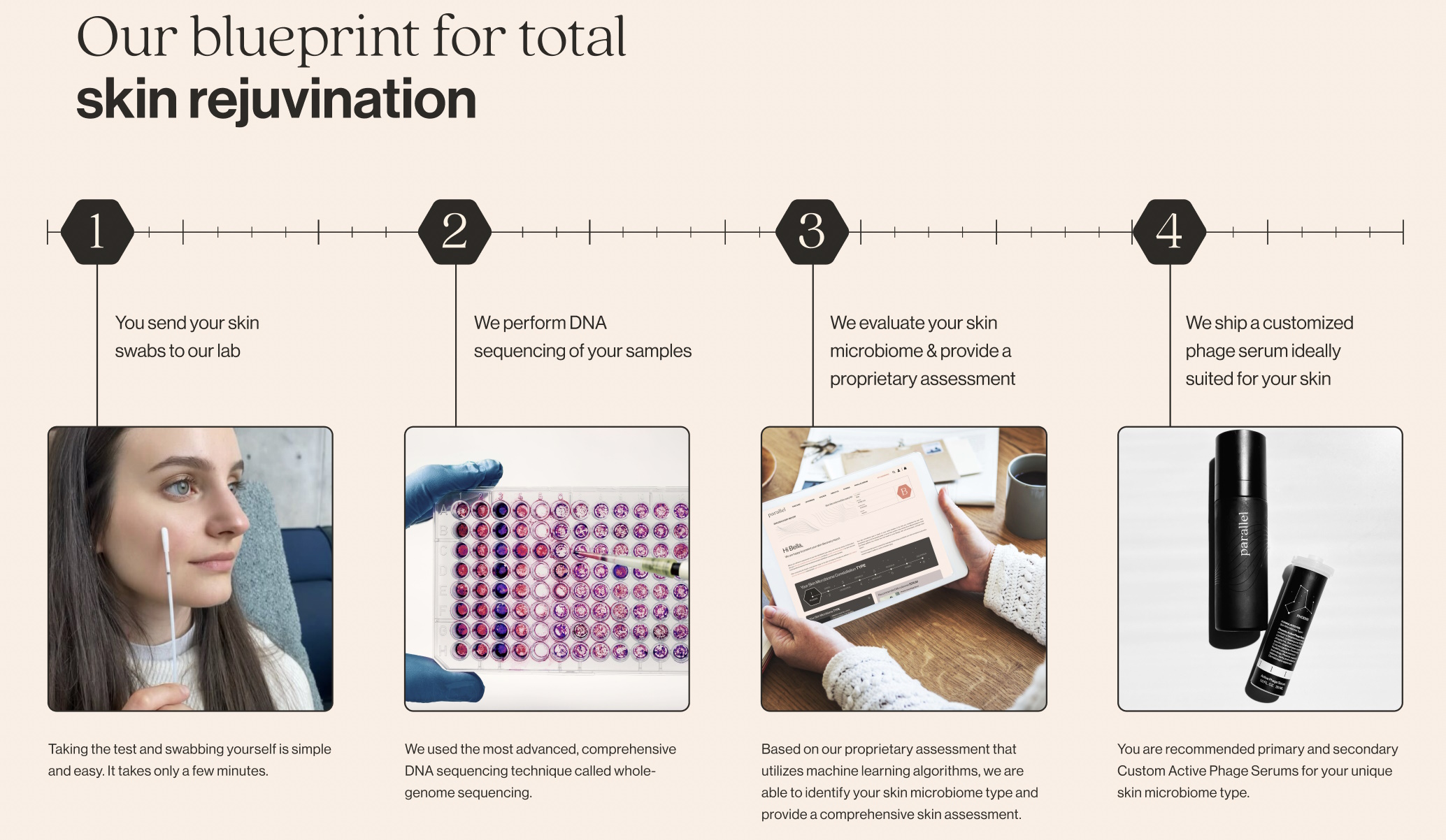
Image Credits:Parallel Health
Fundraising
Gadgets
stake
Image Credits:Tiny Health
Government & Policy
ironware
Layoffs
Media & Entertainment
Meta
Microsoft
privateness
Robotics
security department
Social
blank space
Startups
TikTok
Transportation
speculation
More from TechCrunch
outcome
Startup Battlefield
StrictlyVC
Podcasts
Videos
Partner Content
TechCrunch Brand Studio
Crunchboard
Contact Us
Experts warn of analytical limits, but founders strive to exceed them
Thebiotechsector has embraced the microbiome in recent years , a fleeceable field mart powered by cheap genome sequencing and speculation dollars , predict bespoke handling for everything from gut troubles to skin job . But areport in Sciencealleges that these society miss scientific rigor and meaningful rule , offer little more than guesswork on a complex and understudied area of human wellness . The startups in question pop the question a nuanced answer to this unfavorable judgment that emphasizes their efforts to achieve legitimacy in an area they admit has the potency for snake oil .
The microbiome is a general full term for the alone intermixture of bacteria and other micro-organism that each of us has in and on our bodies . Your skin , gut , mouth , genitals , and any routine of other parts have a fertile diverseness of nonmigratory life , from those thought to be longstanding and beneficial to newer , rare , or even “ invasive ” one .
What many companies have proposed is that by profiling one ’s microbiome , many related to health problems or benefits can be place , treat , or promoted . This profiling is attain by mass genomic depth psychology of a swab , stool sampling , or some other biological source , a process that ( the company say ) quantifies the full , bad , and ugly life endemic to your body .
But six scholars , abduce a variety of researcher , clinician , patient , and other experts they interview , admonish against what they see as a largely unregulated industry rife with snake oil . ( This is not a peer - retrospect research report , but still relies on original enquiry . )
“ These companies claim they can determine whether a customer ’s microbiome is goodly or in ‘ dysbiosis ’ — out of balance — and suggest that if so , it could be the reason for one or more health problems , ” the group writes . “ Some of these companies may wittingly misguide consumer , while most look to engage in questionable practice session that are permitted by disruption in the current regulatory framework . ”
The problem is not with the idea of microbiome profiling in itself . As microbiologist and researchers on microbiome issues , they are well mindful of the potential for , say , an unnatural gut microbiome to lead to any telephone number of wellness problem . The scientist — and entrepreneur — that I ’ve peach to all agree that this is apotentially transformative area of researchand medicine that has only in the last decade become practical to study at plate .
This is because the price of analyzing familial material has dropped through the storey , while the relaxation of handling the large and complicated dataset that results from such examination has risen as tight . The result is an embarrassment of data , in which could very well be shroud a handling route for many a person ’s wellness issues .
Join us at TechCrunch Sessions: AI
Exhibit at TechCrunch Sessions: AI
As investigator pursue links between bacterium and human health , inauguration stand to do good
Where the generator take government issue is with the presentation of microbiome profiling and discussion as launch skill and the want of regulating that prevents them from do these claim .
“ Many of their marketing claim inculpate , and may moderate consumer to think , that the results are strand in scientific accuracy and are medically relevant when that has not been substantiated , ” they write .
Although many company tout studies , these are often internally performed and , because they are based on proprietary data , difficult if not impossible to replicate .
Viome , for illustration , which lift $ 86 million last twelvemonth , cites a studysupporting its title that its inspection and repair can help people with their irritable bowel syndrome , depression , and diabetes . I lack the expertness to evaluate the study , but it and others like it are all by Viome employees , on Viome information , using Viome methods . Though it appear in the American Journal of Lifestyle Medicine , it has not ( like many papers , to be certain ) been cited except by subsequent Viome study . ( I require Viome for comment on the arguments of the Science slice , but did not take heed back as I did from the others . )
It ’s up to you and your medico to decide if this is sufficient scientific financial support for a nutrition - based approach to treating your diabetes . But the Science piece ’s authors assert that all these studies are conducted on wonky grounds , because there is but no take , foundational understanding of microbiomes and their effects on any bodily summons whatsoever .
“ The caller need consistency in process and methods to ensure eubstance across science lab , but they also need consultation standards with which to compare their solvent and check that if they say a consumer ’s results are within a ‘ normal ’ kitchen stove , they are in fact consistent with what would be a ‘ normal ’ range , ” atomic number 27 - author Dianne Hoffman , of the University of Maryland , tell TechCrunch . “ That has not as yet been established . ”
“ There is no consensus about what nominate a goodish human microbiome composition in any population or subpopulation , ” the study say .
Yet microbiome companies tell substance abuser not just what is or is n’t healthy , but also what they can do — and buy — to meliorate it . Nearly half the companies surveyed by the writer sell the supplements they commend , and of course recommend you do a few observe - up trial on their political platform to track their effects .
Even if there were that foundational noesis , the authors channelise out that the testing processes used by microbiome companies “ have been shown to miss analytical validness , resulting in inconsistent test results from the same sampling across unlike laboratories as well as within the same research lab . ” This is not a blanket command that they are inaccurate or that there is nothing company can do to mitigate the tests ’ shortcomings , but that there is no standard and no essential to do so .
The FDA , they note , does not influence these companies , since each is careful about the extent and choice of words of their title and do n’t say that , for example , their supplements are a full - on handling for a disease . or else , they may be described as better outcomes , or advancing holistic well - being , or anything else you might witness on the packaging of a supplement .
The Centers for Medicare & Medicaid Services ( CMS ) nominally regulate them , because their labs must be flow within sure legal limit . But as the author point out , microbiome testing falls between the go because it is not mean to find a picky pathogen or spirit level . They can get CMS certification without showing evidence that their tests are precise . Again , this is no warranty that they are inaccurate , but one must accept it is troubling to hear that so piddling is apparently required of them .
But one may also need , what is wrong with someone receive more information and taking a supplement that , like so many , is at worst uneffective rather than actively harmful ? The report reference a risk of exposure of “ self - misdiagnosis , delay in essay medical treatment , and substituting nonmedical supplements for prescription medicine medications . ”
“ There are patient with chronic bowel illnesses , include children whose parents are searching for therapeutic for their children , who are postdate nutritional or supplement recommendations that are actually harmful to them . One medico we hear from shared that he has heard of patients doing a DIY fecal microbiota organ transplant on the fundament of the consequence from a DTC microbiome based trial , ” Centennial State - author Diane Hoffman recount TechCrunch .
Startups call criticism fair for some, but not for all
The report is unsparing in its unfavorable judgment of this street corner of the manufacture , but it paints with a broad brushwood and by necessity does not delve into specific cases . I asked the leaders of three companies that allow for microbiome examination for various reasons what they think of the affirmation made in the account above .
Natalise Kalea Robinson , Colorado - father and CEO ofParallel Health , which provides bacteriophage - found intervention catering to a client ’s cutis microbiome , in reality hold with many of the points , making it clear that it is up to the troupe to do better .
“ Concerns around analytic cogency are warranted , ” she state . “ This challenge is not only relevant to DTC microbiome companies but to all next - generation diagnostics company at large . When you have technology advancing at the speed that it is , especially spurred by AI , it is strong for regulators to keep up . There is a very real and important hunger among consumer , and within at least some part of the microbiome industry , to in conclusion acquire a clinically validate human microbiome test . That we do not have one already is not a bankruptcy of regulation , but a deficiency of dream in the microbiome industry . ”
The challenge , she said , is that the amount of data require to get a clinically validated , FDA - approved microbiome test think of it ca n’t be take in without a ship’s company like hers doing so as a business . She agreed that there is little consensus on what constitutes a “ healthy ” microbiome and that , as a result , they had to utilise all of their initial funding to ramp up a broad , diverse dataset that would serve as one for their purposes . ( This is still proprietary , interior data , a worry from the account . )
Cheryl Sew Hoy , founder and CEO of Tiny Health , pore on paediatric microbiome data , was flying to point out that while there may be no scientific consensus on adult gut health , there certainly is one for child .
“ Because there are so few germ in the early days — and the only function of the baby intestine in the first 6 months is universally to stand Milk River and not a diversity of food — it ’s much easy from a scientific view to characterise what is sound or unhealthy in an infant ’s gut , which is what Tiny Health particularize in , ” she explained . “ For that reason , the babe gut in the first 1,000 Clarence Day is also much more malleable , only stabilize after 3 years of age . Therefore , there ’s much more scientific establishment of what constitutes a healthy maturation of the infant ’s resistant organization , which is per se tie to the infant ’s bowel development . ”
She said that the company does not name , remedy or treat any weather or ply aesculapian advice . This may be strictly true , but it must be said that it does offer to “ prevent or pull off microbiome - related conditions like colic , eczema , food allergic reaction , asthma attack , stultification and more , ” admittedly with the livelihood of a doctor , but some good word , like exposure to pets , are made in - app . So although they are doing the right matter , they are also flying fairly tight to the Sunday in that sense .
That said , Tiny Health is also laying the origination for becoming an FDA - approved diagnostic and treatment platform , running a pair of clinical work tracking how their reports and recommendation pretend a youngster ’s first 1,000 Day . Sew Hoy also said that they are better termination tracking with strain identification , so you’re able to assure if a particular probiotic is working ( that industry , she noted , is “ currently the barbarian wild westward and not all companies / products are created equal ) .
Daye is amicrobiome startup sharpen on woman ’s generative and intimate wellness , and founder Valentina Milanova was quick to prepare her company asunder from others ( not just because it also has to abide by with U.K. wellness regulations ) .
Flush with Series A funding , Daye unwraps the braggy gynae health commission
“ In the US D2C landscape , the vaginal microbiome is traditionally tested with cistron sequencing technology , which have limitation , when it comes to the standardization and proof of the method acting . These technologies also identify a bit of micro-organism that are largely under - researched and with unknown clinical utility , ” she repoint out . Daye , on the other hand , uses a PCR mental test that identify only a subset of have sex , clinically substantial microorganism . PCR examination also has a more robust validation and timber control system .
Milanova did not disaccord that some microbiomes are under - documented , but said the vaginal microbiome is not one of them . “ There is a scientific consensus on what constitutes a healthy vaginal microbiome — one that is command by lactobacilli bacteria . These produce lactic acid and other antimicrobic center that maintain a protective vaginal pH and inhibit timeserving bacterium from rise and causing contagion , ” she wrote .
“ Daye ’s trial enables us to measure the comparative copiousness of good bacterium , as well as that of opportunistic micro-organism , that do BV and yeast infections when find in large total , ” she continued , but this is only one footfall in a clinician - involved operation that must place the specific pathogen before recommending a treatment . In the U.K. , this can be done within Daye ’s platform , but in the U.S. , they work with a web of clinicians .
She also shared Kalea Robinson ’s perspective that venture funding is “ an imperfect chemical mechanism for funding R&D and creation … venture capital investors operate on a short timeline and venture - backed startups involve to furnish commercial results in timeline , which are often unreasonable for aesculapian excogitation . ” They use concession and regular income as well to stomach this important work .
As far as regularisation , all three women said they receive it , as it should serve secure reproducible and helpful outcome .
“ We trust that the FDA will recognize that microbiome tests can help improve wellness outcome if done right on , ” Kalea Robinson pronounce . She look its authority to expand and include their oeuvre sooner or later , and to that death “ we are doing the difficult work now ” to prepare for that .
For those that are n’t , the sentiency is that regulation is needed to curtail this nascent and disorganized diligence ’s equivalent weight of invasive and opportunistic microorganism — even the well - funded ace .